Predictors of Response to Hydroxyurea and Switch to Ruxolitinib in HU-Resistant Polycythaemia VERA Patients: A Real-World PV-NET Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Definitions
2.3. Ethical Aspects
2.4. Statistical Analyses
2.5. Data Sharing Statement
3. Results
3.1. Study Cohort
3.2. Efficacy and Safety of Hydroxyurea
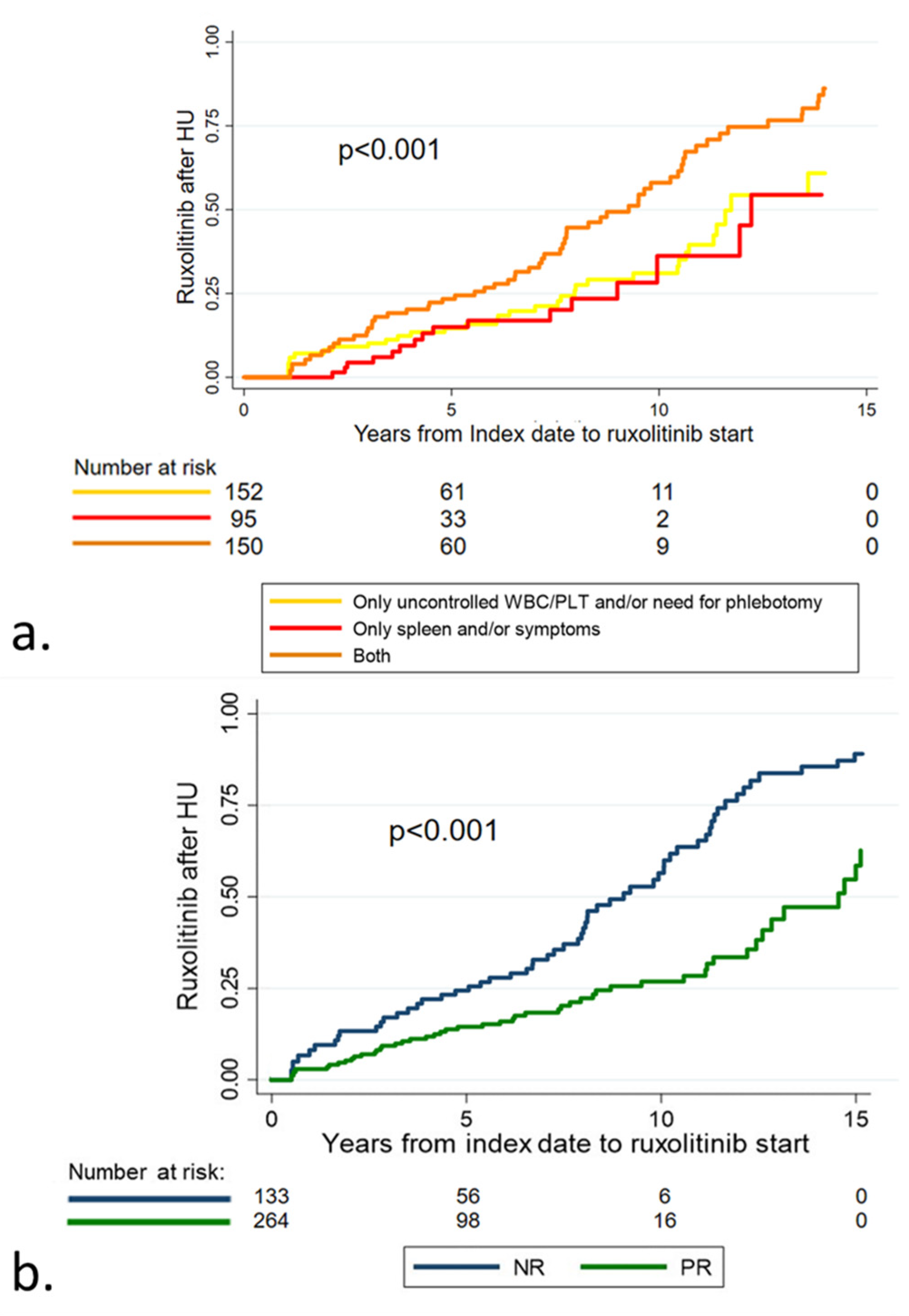
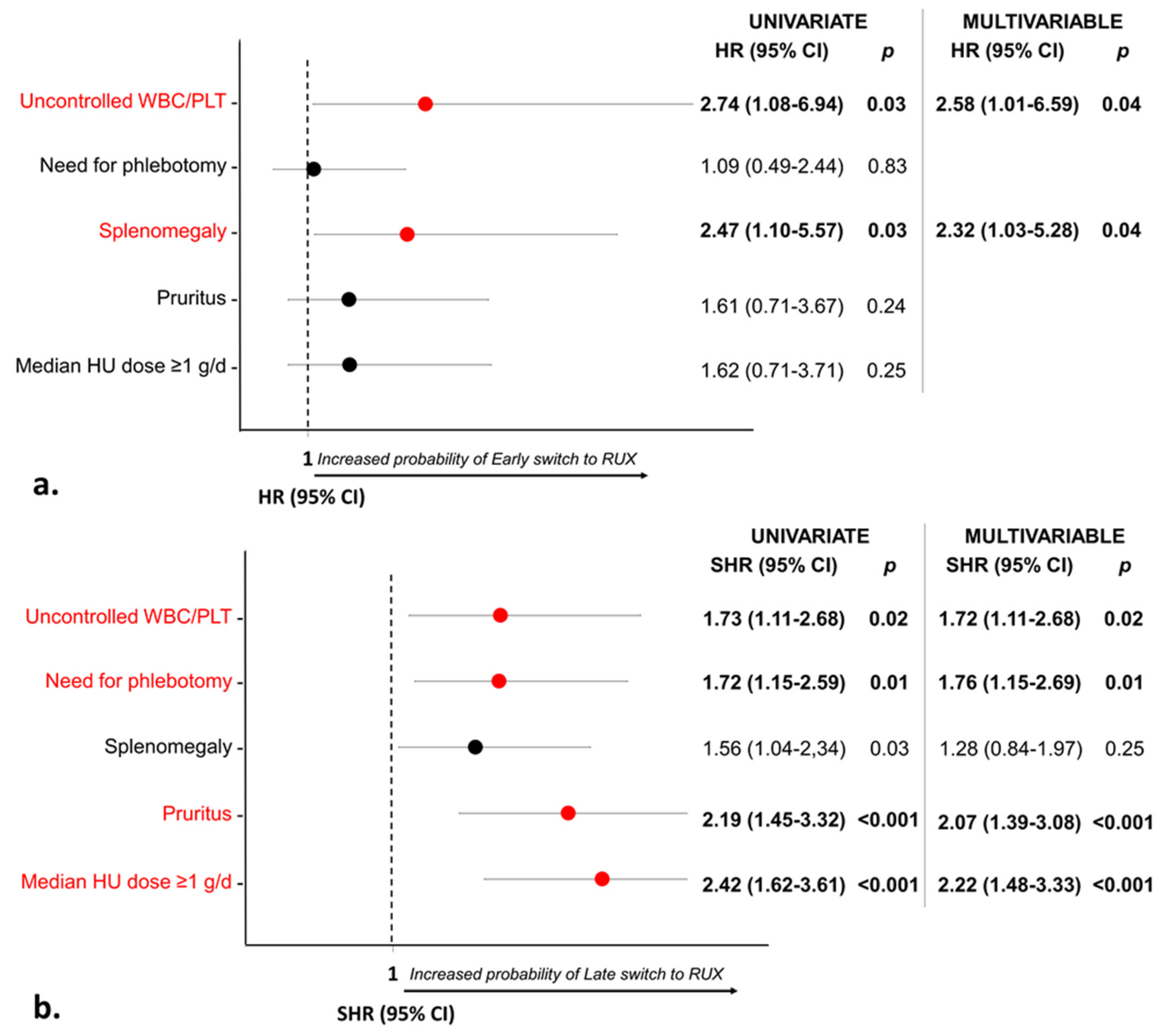
3.3. Treatment Strategy in Patients with Stable Poor Response
3.4. Outcome according to Response to Hydroxyurea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Center | Contributors |
| IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy / Dipartimento di Medicina Specialistica, Diagnostica e Sperimentale, Università di Bologna, Bologna, Italy | Francesca Palandri Giuseppe Auteri Camilla Mazzoni Simona Paglia Nicola Vianelli Michele Cavo |
| Section of Hematology, Department of Radiological and Hematological Sciences, Catholic University School of Medicine/Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy | Elena Rossi Silvia Betti Francesco Ramundo Sara Ceglie Valerio De Stefano |
| Division of Cellular Biotechnologies and Hematology, University Sapienza, Rome, Italy | Massimo Breccia Emilia Scalzulli Gioia Colafigli |
| Division of Hematology, Città della Salute e della Scienza Hospital, Torino, Italy | Giulia Benevolo Francesca Pirillo |
| HematologyDivision, San Gerardo Hospital, ASST Monza, Italy | Elena M. Elli Alessia Ripamonti |
| Division of Hematology, University of Ferrara, Italy | Francesco Cavazzini Antonio Cuneo |
| Unit of Hematology and Clinical Immunology, University of Padova, Padova, Italy | Gianni Binotto Gianpietro Semenzato Fabio D’Amore Antonio Maroccia |
| Department of Hematology, Azienda USL—IRCCS di Reggio Emilia, Reggio Emilia, Italy | Alessia Tieghi Katia Codeluppi Domenico Penna Elisabetta Lugli |
| Division of Hematology and BMT, Azienda Sanitaria Universitaria Integrata di Udine, Italy | Mario Tiribelli Rikard Mullai Umberto Pizzano |
| Innere Medicine C, Universitätsmedizin Greifswald, Greifswald, Germany | Florian H. Heidel |
| Section of Hematology, University of Verona, Verona, Italy | Massimiliano Bonifacio Mauro Krampera Luigi Scaffidi Andrea Bernardelli |
| Department of Clinical Medicine and Surgery, Federico II University Medical School, Naples, Italy | Novella Pugliese Fabrizio Pane |
| Ematologia, Ospedale Businco, Università degli studi di Cagliari, Cagliari, Italy | Giovanni Caocci Maria Pina Simula Olga Mulas Alessandro Costa |
| Division of Hematology, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy | Monica Crugnola Elena Masselli |
| Unit of Hematology, Hospital of Cosenza, Cosenza, Italy | Francesco Mendicino Enrica A. Martino |
| Division of Hematology, Onco-hematologic Department, AUSL della Romagna, Ravenna | Alessandra D’Addio Francesco Lanza |
| Hematology Unit, Infermi Hospital Rimini, Rimini | Simona Tomassetti |
| Division of Hematology, Azienda Ospedaliera ‘Bianchi Melacrino Morelli’, Reggio Calabria, Italy | Bruno Martino |
| Unit of Blood Diseases and Stem Cell Transplantation, ASST Spedali Civili di Brescia, Brescia, Italy | Nicola Polverelli Domenico Russo Lisa Gandolfi |
| IRCCS Policlinico San Martino, Genova, Italy/Clinic of Hematology, Department of Internal Medicine (DiMI), Genova, Italy | Roberto M. Lemoli Maurizio Miglino Maria Grazia Ciardo |
| Department of Scienze Mediche, Chirurgiche e Tecnologie Avanzate “G.F. Ingrassia”, University of Catania, Italy | Giuseppe A. Palumbo Bruno Garibaldi Ernesta Lambusta |
| Hematology, Fabrizio Spaziani Hospital, Frosinone, Italy | Alessandro Andriani |
| Hematology Unit, Ospedale Belcolle, Viterbo, Italy | Roberto Latagliata |
References
- Marchioli, R.; Finazzi, G.; Landolfi, R.; Kutti, J.; Gisslinger, H.; Patrono, C.; Marilus, R.; Villegas, A.; Tognoni, G.; Barbui, T. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J. Clin. Oncol. 2005, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Scherber, R.; Kosiorek, H.; Dueck, A.C.; Kiladjian, J.-J.; Xiao, Z.; Slot, S.; Zweegman, S.; Sackmann, F.; Fuentes, A.K.; et al. Symptomatic Profiles of Patients With Polycythemia Vera: Implications of Inadequately Controlled Disease. J. Clin. Oncol. 2016, 34, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Polycythemia vera: Historical oversights, diagnostic details, and therapeutic views. Leukemia 2021, 35, 3339–3351. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, A.M. From leeches to personalized medicine: Evolving concepts in the management of polycythemia vera. Haematologica 2017, 102, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, M.; Vannucchi, A.M.; Griesshammer, M.; Harrison, C.; Koschmieder, S.; Gisslinger, H.; Álvarez-Larrán, A.; De Stefano, V.; Guglielmelli, P.; Palandri, F.; et al. Appropriate management of polycythaemia vera with cytoreductive drug therapy: European LeukemiaNet 2021 recommendations. Lancet Haematol. 2022, 9, e301–e311. [Google Scholar] [CrossRef]
- Alvarez-Larrán, A.; Pereira, A.; Cervantes, F.; Arellano-Rodrigo, E.; Hernández-Boluda, J.-C.; Ferrer-Marín, F.; Angona, A.; Gómez, M.; Muiña, B.; Guillén, H.; et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood 2012, 119, 1363–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Larrán, A.; Kerguelen, A.; Hernández-Boluda, J.C.; Pérez-Encinas, M.; Ferrer-Marín, F.; Bárez, A.; Martínez-López, J.; Cuevas, B.; Mata, M.I.; García-Gutiérrez, V.; et al. Frequency and prognostic value of resistance/intolerance to hydroxycarbamide in 890 patients with polycythaemia vera. Br. J. Haematol. 2016, 172, 786–793. [Google Scholar] [CrossRef] [Green Version]
- Randi, M.L.; Ruzzon, E.; Luzzatto, G.; Tezza, F.; Girolami, A.; Fabris, F. Safety profile of hydroxyurea in the treatment of patients with Philadelphia-negative chronic myeloproliferative disorders. Haematologica 2005, 90, 261–262. [Google Scholar]
- Antonioli, E.; Guglielmelli, P.; Pieri, L.; Finazzi, M.; Rumi, E.; Martinelli, V.; Vianelli, N.; Randi, M.L.; Bertozzi, I.; De Stefano, V.; et al. Hydroxyurea-related toxicity in 3,411 patients with Ph′-negative MPN. Am. J. Hematol. 2012, 87, 552–554. [Google Scholar] [CrossRef]
- Barosi, G.; Birgegard, G.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.; Kiladijan, J.-J.; Lengfelder, E.; Mesa, R.; Mc Mullin, M.F.; et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: Results of a European LeukemiaNet (ELN) consensus process. Br. J. Haematol. 2010, 148, 961–963. [Google Scholar] [CrossRef]
- Barosi, G.; Mesa, R.; Finazzi, G.; Harrison, C.; Kiladjian, J.-J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; Vannucchi, A.M.; Besses, C.; et al. Revised response criteria for polycythemia vera and essential thrombocythemia: An ELN and IWG-MRT consensus project. Blood 2013, 121, 4778–4781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vannucchi, A.M.; Kiladjian, J.J.; Griesshammer, M.; Masszi, T.; Durrant, S.; Passamonti, F.; Harrison, C.N.; Pane, F.; Zachee, P.; Mesa, R.; et al. Ruxolitinib versus Standard Therapy for the Treatment of Polycythemia Vera. N. Engl. J. Med. 2015, 372, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiladjian, J.J.; Zachee, P.; Hino, M.; Pane, F.; Masszi, T.; Harrison, C.N.; Mesa, R.; Miller, C.B.; Passamonti, F.; Durrant, S.; et al. Long-term efficacy and safety of ruxolitinib versus best available therapy in polycythaemia vera (RESPONSE): 5-year follow up of a phase 3 study. Lancet Haematol. 2020, 7, e226–e237. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Griesshammer, M.; Palandri, F.; Egyed, M.; Benevolo, G.; Devos, T.; Callum, J.; Vannucchi, A.M.; Sivgin, S.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): A randomised, open-label, phase 3b study. Lancet Oncol. 2017, 18, 88–99. [Google Scholar] [CrossRef]
- Griesshammer, M.; Saydam, G.; Palandri, F.; Benevolo, G.; Egyed, M.; Callum, J.; Devos, T.; Sivgin, S.; Guglielmelli, P.; Bensasson, C.; et al. Ruxolitinib for the treatment of inadequately controlled polycythemia vera without splenomegaly: 80-week follow-up from the RESPONSE-2 trial. Ann. Hematol. 2018, 97, 1591–1600. [Google Scholar] [CrossRef] [Green Version]
- Passamonti, F.; Palandri, F.; Saydam, G.; Callum, J.; Devos, T.; Guglielmelli, P.; Vannucchi, A.M.; Zor, E.; Zuurman, M.; Gilotti, G.; et al. Ruxolitinib versus best available therapy in inadequately controlled polycythaemia vera without splenomegaly (RESPONSE-2): 5-year follow up of a randomised, phase 3b study. Lancet Haematol. 2022, 9, e480–e492. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2008. [Google Scholar]
- Barosi, G.; A Mesa, R.; Thiele, J.; Cervantes, F.; Campbell, P.J.; Verstovsek, S.; Dupriez, B.; Levine, R.L.; Passamonti, F.; Gotlib, J.; et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: A consensus statement from the international working group for myelofibrosis research and treatment. Leukemia 2008, 22, 437–438. [Google Scholar] [CrossRef] [Green Version]
- Barosi, G.; Birgegard, G.; Finazzi, G.; Griesshammer, M.; Harrison, C.; Hasselbalch, H.C.; Kiladjian, J.J.; Lengfelder, E.; McMullin, M.F.; Passamonti, F.; et al. Response criteria for essential throm-bocythemia and polycythemia vera: Result of a European LeukemiaNet consensus conference. Blood 2009, 113, 4829–4833. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.W.; Vasan, R.S. Cohort Profile: The Framingham Heart Study (FHS): Overview of milestones in cardiovascular epidemi-ology. Int. J. Epidemiol. 2015, 44, 1800–1813. [Google Scholar] [CrossRef] [Green Version]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jentsch-Ullrich, K.; Eberhardt, J.; Zeremski, V.; Koehler, M.; Wolleschak, D.; Heidel, F.H. Characteristics and treatment of polycythemia vera patients in clinical practice: A multicenter chart review on 1476 individuals in Germany. J. Cancer Res. Clin. Oncol. 2016, 142, 2041–2049. [Google Scholar] [CrossRef]
- Marchioli, R.; Finazzi, G.; Specchia, G.; Masciulli, A.; Mennitto, M.R.; Barbui, T. The CYTO-PV: A Large-Scale Trial Testing the Intensity of CYTOreductive Therapy to Prevent Cardiovascular Events in Patients with Polycythemia Vera. Thrombosis 2011, 2011, 794240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, C.N.; Nangalia, J.; Boucher, R.; Jackson, A.; Yap, C.; O′Sullivan, J.; Fox, S.; Ailts, I.; Dueck, A.C.; Geyer, H.L.; et al. Ruxolitinib Versus Best Available Therapy for Polycythemia Vera Intolerant or Resistant to Hydroxycarbamide in a Randomized Trial. J. Clin. Oncol. 2023, 41, 3534–3544. [Google Scholar] [CrossRef]
- Ronner, L.; Podoltsev, N.; Gotlib, J.; Heaney, M.L.; Kuykendall, A.T.; O′Connell, C.; Shammo, J.; Fleischman, A.G.; Scherber, R.M.; Mesa, R.; et al. Persistent leukocytosis in polycythemia vera is associated with disease evolution but not thrombosis. Blood 2020, 135, 1696–1703. [Google Scholar] [CrossRef]
- Tremblay, D.; Srisuwananukorn, A.; Ronner, L.; Podoltsev, N.; Gotlib, J.; Heaney, M.L.; Kuykendall, A.; O’connell, C.L.; Shammo, J.M.; Fleischman, A.; et al. European LeukemiaNet Response Predicts Disease Progression but Not Thrombosis in Polycythemia Vera. Hemasphere 2022, 6, e721. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Complete Responders (n. 166) | Poor Responders (n. 397) | p Value |
|---|---|---|---|
| Age, median (range), years Age ≥ 65 years | 70 (47–87) 116 (69.9%) | 65 (21–89) 206 (51.9%) | <0.001 <0.001 |
| Male sex, no. (%) | 66 (39.8%) | 220 (55.4%) | 0.001 |
| JAK2V617F VAF ≥ 50%, no. (%) on 365 evaluable | 45/114 (39.5%) | 135/251 (53.8%) | 0.01 |
| Platelet count, median (range), ×109/L | 500 (159–1279) | 449 (138–1209) | 0.004 |
| Leukocytes, median (range), ×109/L | 10 (3.3–30.3) | 10.1 (1–27.3) | 0.70 |
| Hemoglobin, median (range), g/dL | |||
| Male | 18.6 (15.8–23.6) | 18.7 (12–23.4) | 0.59 |
| Female | 17.8 (15.3–22) | 17.5 (13.2–21.9) | 0.09 |
| Hematocrit, median (range), % | |||
| Male | 55 (48.9–72.5) | 56.3 (38–73) | 0.70 |
| Female | 54 (47.6–71.7) | 54.1 (39–72) | 0.99 |
| Palpable spleen, no. (%) of 548 evaluable | 26/165 (15.8%) | 151/383 (39.4%) | <0.001 |
| Pruritus, no. (%) | 28 (17%) | 158 (39.9%) | <0.001 |
| BMI ≥ 25, % of 349 evaluable | 32/65 (49.2%) | 144/284 (50.0%) | 0.83 |
| At least one CVRF, no. (%) | 129 (77.7%) | 312 (78.6%) | 0.81 |
| Thromboses pre-/at diagnosis, no. (%) | 39 (23.5%) | 102 (25.7%) | 0.58 |
| Median HU dose, median (range), g/d of 506 evaluable Median HU dose ≥ 1 g/d, no. (%) | 0.8 (0.2–2) 59/119 (49.6%) | 0.5 (0.2–2) 101/387 (26.17%) | <0.001 <0.001 |
| Toxicities | HU < 1 g/d (n. 346) | HU ≥ 1 g/d (n. 160) | p | ||
|---|---|---|---|---|---|
| n. (%) | Incidence Rate (per 100 Patient-Years) | n. (%) | Incidence Rate (per 100 Patient-Years) | ||
| Hematological toxicity | 22 (6.4%) | 1.7 | 26 (16.3%) | 4.0 | 0.003 |
| Anemia | 5 (1.5%) | 0.4 | 9 (5.7%) | 1.3 | 0.03 |
| Thrombocytopenia | 15 (4.3%) | 1.2 | 16 (10%) | 2.5 | 0.09 |
| Neutropenia | 2 (0.6%) | 0.1 | 1 (0.6%) | 0.2 | 1.0 |
| Extra-hematological toxicity | 42 (12.1%) | 3.1 | 33 (20.6%) | 4.7 | 0.11 |
| Skin ulcers | 18 (5.2%) | 1.4 | 20 (12.5%) | 2.9 | 0.02 |
| Oral aftosis | 9 (2.6%) | 0.7 | 4 (2.5%) | 0.6 | 0.81 |
| Gastro-intestinal disturbances | 4 (1.1%) | 0.3 | 3 (1.9%) | 0.4 | 0.65 |
| Fever | 2 (0.6%) | 0.1 | 0 | 0 | 0.43 |
| Myalgia | 2 (0.6%) | 0.1 | 0 | 0 | 0.43 |
| Zoster reactivations | 1 (0.3%) | 0.1 | 1 (0.6%) | 0.2 | 0.69 |
| Non-melanoma skin cancer | 6 (1.7%) | 0.4 | 5 (3.1%) | 0.6 | 0.53 |
| Overall toxicity | 64 (18.5%) | 4.8 | 59 (36.9%) | 8.7 | 0.002 |
| HU-CR (n.166) | HU-POOR (n.283) | HU-CR versus HU-POOR | ||||
|---|---|---|---|---|---|---|
| Exposure (patient-years) | 717.4 | 1182.4 | ||||
| Events | Total n. | n. | Incidence rate (per 100 patient-years) | n. | Incidence rate (per 100 patient-years) | p |
| Thromboses Arterial Venous | 51 24 27 | 16 9 7 | 2.36 1.33 1.03 | 25 11 14 | 2.23 0.98 1.25 | 0.47 |
| Haemorrhages | 25 | 13 | 1.45 | 12 | 0.84 | 0.17 |
| Infections Lung Mucocutaneous Urinary tract Herpes zoster Herpes simplex Gastrointestinal Sepsis Other | 43 14 7 5 7 3 3 2 2 | 17 8 1 2 5 0 1 0 0 | 2.69 1.26 0.16 0.32 0.79 0 0.16 0 0 | 17 4 4 2 1 1 2 2 1 | 1.53 0.36 0.36 0.18 0.09 0.09 0.18 0.18 0.09 | 0.06 |
| Second primary malignancy Non-melanoma skin cancer Squamous cell carcinoma Malignant melanoma Prostate Breast Lung Gastrointestinal Lymphoma Other | 52 12 4 2 8 7 4 5 1 9 | 19 6 0 0 2 4 2 1 1 3 | 2.88 0.92 0 0 0.30 0.61 0.30 0.15 0.15 0.45 | 30 5 4 1 5 3 2 4 0 6 | 2.74 0.45 0.37 0.09 0.45 0.28 0.18 0.37 0 0.55 | 0.34 |
| Death | 35 | 15 | 1.62 | 20 | 1.36 | 0.59 |
| Blast Phase | 9 | 5 | 0.71 | 4 | 0.34 | 0.14 |
| Post-PV myelofibrosis | 14 | 5 | 0.72 | 9 | 0.78 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palandri, F.; Rossi, E.; Auteri, G.; Breccia, M.; Paglia, S.; Benevolo, G.; Elli, E.M.; Cavazzini, F.; Binotto, G.; Tieghi, A.; et al. Predictors of Response to Hydroxyurea and Switch to Ruxolitinib in HU-Resistant Polycythaemia VERA Patients: A Real-World PV-NET Study. Cancers 2023, 15, 3706. https://doi.org/10.3390/cancers15143706
Palandri F, Rossi E, Auteri G, Breccia M, Paglia S, Benevolo G, Elli EM, Cavazzini F, Binotto G, Tieghi A, et al. Predictors of Response to Hydroxyurea and Switch to Ruxolitinib in HU-Resistant Polycythaemia VERA Patients: A Real-World PV-NET Study. Cancers. 2023; 15(14):3706. https://doi.org/10.3390/cancers15143706
Chicago/Turabian StylePalandri, Francesca, Elena Rossi, Giuseppe Auteri, Massimo Breccia, Simona Paglia, Giulia Benevolo, Elena M. Elli, Francesco Cavazzini, Gianni Binotto, Alessia Tieghi, and et al. 2023. "Predictors of Response to Hydroxyurea and Switch to Ruxolitinib in HU-Resistant Polycythaemia VERA Patients: A Real-World PV-NET Study" Cancers 15, no. 14: 3706. https://doi.org/10.3390/cancers15143706
APA StylePalandri, F., Rossi, E., Auteri, G., Breccia, M., Paglia, S., Benevolo, G., Elli, E. M., Cavazzini, F., Binotto, G., Tieghi, A., Tiribelli, M., Heidel, F. H., Bonifacio, M., Pugliese, N., Caocci, G., Crugnola, M., Mendicino, F., D'Addio, A., Tomassetti, S., ... De Stefano, V. (2023). Predictors of Response to Hydroxyurea and Switch to Ruxolitinib in HU-Resistant Polycythaemia VERA Patients: A Real-World PV-NET Study. Cancers, 15(14), 3706. https://doi.org/10.3390/cancers15143706















